Energy in Radiation
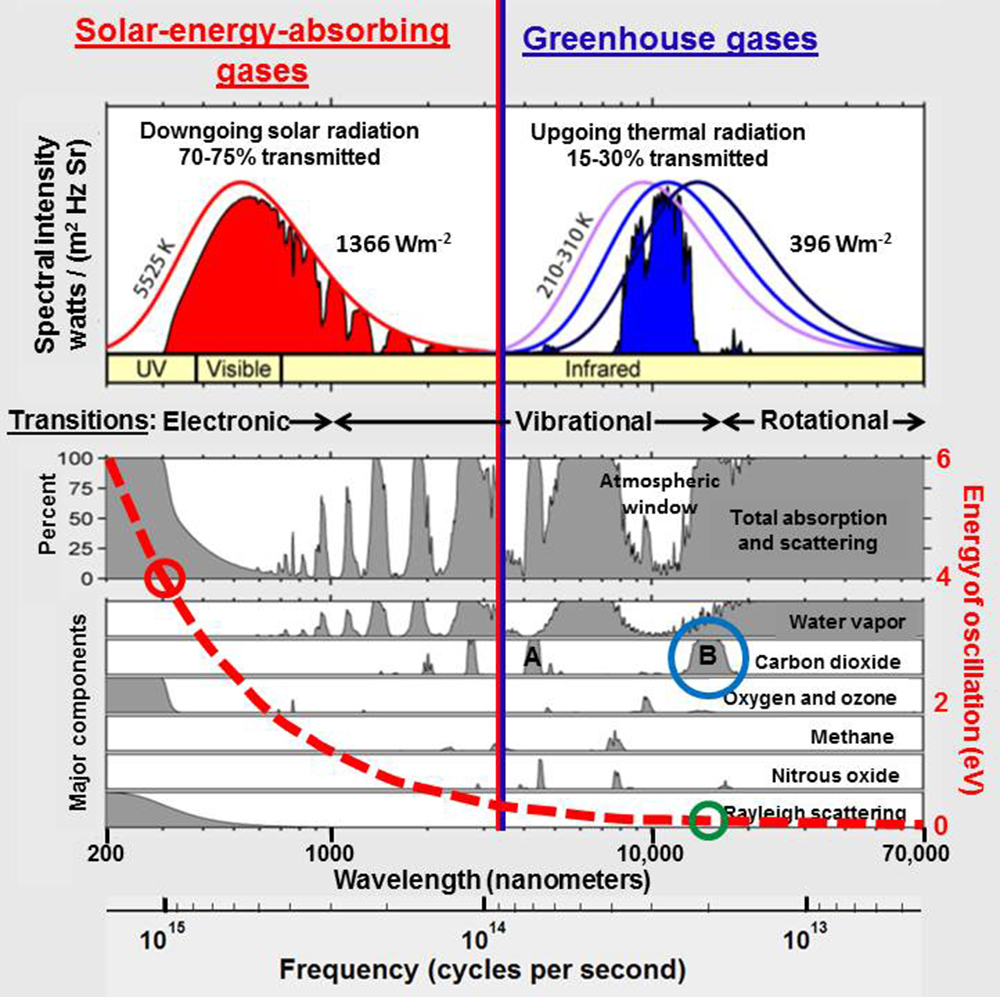
Greenhouse gases such as carbon dioxide and methane absorb only a very small amount of the broad spectrum of infrared heat (energy) radiated by Earth. Based on a figure by Rohde (2007).
The energy of radiation absorbed by carbon dioxide around 14,900 nanometers (blue circle) is near 0.08 electronvolts (green circle) while the energy that reaches Earth when the ozone layer is depleted around 310 nanometers (red circle) is near 4 electronvolts, 48 times larger. The red dashed line shows the Planck postulate (E=hν) emphasizing that the energy contained in radiation increases with decreasing wavelength, increasing frequency.
The solid red line shows the spectral radiance, the amount of radiation at each wavelength, for solar radiation at the top of Earth’s atmosphere, according to Planck’s law, assuming the Sun has a surface temperature of 5525K. The purple, blue, and navy lines show the spectral radiance for infrared energy radiated by Earth at a temperature of 210K, 260K, and 310K respectively. The solar and terrestrial curves have been normalized to have the same height. Peak spectral radiance of solar radiation at the top of the atmosphere is actually 69 times larger than the peak spectral radiance for terrestrial radiation. The blue shaded area shows the spectral radiance for terrestrial radiation reaching space while the red shaded area shows the spectral radiance for solar radiation reaching Earth.
The gray shaded areas show the percentage of absorption and Rayleigh scattering that occur at each wavelength. Note that water vapor absorbs energy over a wide variety of wavelengths while carbon dioxide only absorbs energy in a few narrow bandwidths. The bandwidth around 4,300 nanometers (marked A) does not overlap with a water vapor absorption band but contains little infrared energy. The bandwidth around 14,900 nanometers (marked B) overlaps absorption by water vapor substantially, although the spectral lines (Rothman et al., 2013) for the two gases do not overlap much in detail. Concentrations of water vapor range from 0.1 to 5% while concentrations of CO2 are less than 0.04%.
Note that absorption by photodissociation of ozone is along a continuum at wavelengths below 0.35 μm and that much of what we think of as Rayleigh scattering my be related to photodissociation.